Introduction
Magnetic resonance imaging (MRI) allows for non-invasive and longitudinal characterization of brain anatomy, presence of abnormalities, and neurodegeneration.1,2 Structural MRIs enable volumetric quantification of brain structures as well as tumors and lesions, while diffusion-weighted and quantitative MRIs provide valuable information on microstructure and biophysical properties of the tissue.3,4 While MRI measures are sensitive to neurodegenerative processes and brain abnormalities, they are not specific. There is therefore a key need to supplement, validate, and characterize morphological measures and biophysical models with neurobiological information. Complementing in vivo MRI assessments, post-mortem MRI can be performed at longer sessions (i.e. overnight) with higher resolutions and be linked to microscopy assessments, allowing for multi-scale investigations of the brain.5–7 Finally, ex vivo MRI measurements can be linked to post-mortem neuropathology assessments to develop potential MRI-based neuropathology biomarkers.8,9 For example, ex vivo transverse relaxation rate (R2*) is sensitive to the presence of amyloid plaques in aging and Alzheimer’s disease specimens.10–12 Quantitative susceptibility mapping (QSM), on the other hand, is highly sensitive and specific to the presence of local diamagnetic (e.g. myelin) and paramagnetic (e.g. iron deposits) signal sources.13 Combined, multi-modal ex vivo quantitative MRI can provide a multidimensional view of the underlying tissue properties, which can advance our understanding of its pathological changes.
Neuropathology confirmation of in vivo brain MRI findings requires post-mortem assessment of the same regions through histology, which can be achieved by mapping the in vivo MRIs to the histology section images.14–18 Histology is usually performed on smaller tissue blocks that need to be mapped back to the whole brain MRI.9 To facilitate this process, the histology blocks can be scanned (preferably using ultra-high field 7T scanners to achieve higher image resolution), providing a reliable intermediate image that can be accurately mapped to both in vivo MRIs and histology images.15,19–21 Ex vivo MRIs of intact brains can also be used to accurately localize areas of interest for further histology and immunohistochemistry (IHC) assessments (i.e. presence of cerebrovascular lesions such as white matter hyperintensities, tumors, etc.) without cutting into the tissue.
In this work, we present a post-mortem MRI protocol that has been developed and optimized for the Douglas-Bell Canada Brain Bank (DBCBB) specimens, allowing for data acquisition from a large number of post-mortem brains without jeopardizing tissue quality for follow-up histology and immunohistochemistry assessments. We demonstrate feasibility of performing high quality post-mortem MRIs with a standard one hour protocol using a 3T human MRI scanner, as well as two higher resolution overnight protocols using the same 3T scanner as well as a 7T preclinical scanner for achieving greater levels of anatomical detail.
Methods
Post-mortem Specimens: The DBCBB is one of the largest brain banks worldwide, housing over 3000 brains with different neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, Amyotrophic lateral sclerosis (ALS), as well as diverse psychiatric conditions including schizophrenia, major depression, bipolar disorder, substance use disorders (https://douglasbrainbank.ca/). Linked to a large relational database of demographics, family history, and neuropathology information, the DBCBB specimens provide an invaluable resource for studying the neurobiology of brain diseases. Brain specimens are collected post-mortem in accordance with informed consent of the donors or the next of kin, according to tissue banking practices regulated by the Quebec Health Research Fund, and the Guidelines on Human Biobanks and Genetic Research Databases, overseen by the Douglas Research Ethics Board.22
After reception at the DBCBB, hemispheres are separated by a sagittal cut in the middle of the brain, brainstem, and cerebellum. One unsliced hemisphere (right or left, in alternation) is fixed in 10% neutral buffered formalin (in 7-liter containers, immersed in 6 liters of formalin) to prevent tissue decomposition. After three weeks of fixation, the hemispheres are transferred to and remain in air-tight MRI-compatible plastic containers fully immersed in 10% neutral buffered formalin. The brains are scanned in the same plastic containers to minimize tissue manipulation and prevent MRI artifacts caused by the presence of air bubbles in the ventricles and sulci due to exposure of tissue to air, which can in turn negatively impact the quality of the MRI acquisition, in particular for quantitative MRI sequences. The specimens that need to be scanned prior to the three-week timepoint are transferred to the same containers for scanning, and returned to the larger containers after the scan is completed. Tissue soaking in PBS is not performed as previous studies have reported inconsistent fluid penetration for whole brain specimens, creating artificial boundaries in the resulting images.7,23
The intact brain hemispheres are scanned using the 3T Siemens Prisma Fit human MRI scanner at the Douglas Cerebral Imaging Centre (CIC). The containers are stabilized in the 64-channel head/neck coil, with the hemispheres lying flat on the sagittal cut to minimize tissue motion and drifting during the scan and with the cerebellum towards in-bore to standardize the positioning and facilitate future application of automated image processing tools. The tissue is positioned in the center of the container (i.e. not touching the sides) to prevent potential artifacts. Figure 1 demonstrates the protocol for preparation of the tissue for scanning and positioning of the hemispheres in the 3T MRI scanner. Table 1 summarizes the 3T acquisition protocol used to acquire the post-mortem images. All acquisitions were performed with one average.
For B1+ variations, we used the sequence available by the vendor (Siemens, for softwares above V11), consisting of a turbo fast-low-angle-shot sequence with centric k-space reordering applied immediately after a slice selective preconditioning pulse.24 This sequence is faster than other B1+ mapping acquisition methods and has been used in Multiparameter Mapping (MPM) protocols.25 Three GRE echoes are also acquired based on the variable flip angle method, to be used for mapping T1 and T2*. They consist of a multi-echo gradient echo sequence, repeated three times with different flip angles. Finally, phase unwrapping is performed to calculate QSM maps, for which we used a multi-echo-gradient-echo sequence with 12 echoes.
A subset of the specimens is also scanned using an overnight 15-hour version of the same protocol, with higher resolutions and more averages, to provide additional neuroanatomical details. Table 2 summarizes the overnight 3T acquisition protocol.
Following the completion of the fixation process, smaller tissue blocks are extracted and scanned at higher resolution with an overnight 15-hour protocol using the 7T Bruker Biospec animal scanner at the Douglas CIC using comparable sequences. Table 3 summarizes the 7T acquisition protocol used to acquire the post-mortem images. Consistent with the 3T protocol, the tissue blocks are scanned immersed in 10% formalin in MRI-compatible plastic containers. A 3D-printed holder is used to stabilize the container inside the 7T MRI coil to minimize tissue motion and drifting.
Image Processing
The open access MINC toolkit V2 Version 1.9.18 (https://bic-mni.github.io/) was used to perform preprocessing, longitudinal co-registration of the T1w images, registration to stereotaxic space, and registration of the tissue block MRI to the hemisphere MRI.26 BISON tissue classification tool was used to generate brain masks for quantitative and diffusion MRI processing.27 To improve the processing of the diffusion images, two fast series of DWI with b = zero are acquired, using the DWI phase encoding (right-left) and the reverse (left-right). MRtrix3 (Version 3.0.4)28 was used to denoise DWI volumes and to correct ringing artifacts. The mrtrix3 wrapper dwifslpreproc for FMRIB Software Library (FSL v6)29,30 was then used to correct eddy-currents and susceptibility distortions, and the output was bias corrected using the ANTs N4 wrapper dwibiascorrect.31,32 The tensor model (DTI) was then computed via Mrtrix3, and Fractional Anisotropy (FA) and Mean Diffusivity (MD) maps were extracted. We further performed probabilistic tractography on the preprocessed DWI volumes, seeding from the brainstem and terminating in the primary motor cortex using Mrtrix3’s tckgen. qMRLab was used to generate quantitative T1 and T2* maps (based on MGE-VFA acquisitions) as well as T2 and Myelin Water Fraction (MWF) maps (based on 3D meGSE acquisitions) from the quantitative MRI data.33 Morphology Enabled Dipole Inversion approach (MEDI) toolbox was used to generate QSM maps based on the 3D multi-echo gradient echo sequence.34,35
Results
Sample Characteristics
The data presented in this section represents the characteristics of the post-mortem images (N ~ 200) acquired at the Douglas CIC between September 2022 and December 2023. To ensure the feasibility of the developed protocols across age ranges and disorders, post-mortem scans were performed in brains with a range of disorders, including Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontotemporal dementia, vascular dementia, mixed dementias, ALS, depressed suicides, as well as age-matched controls without any neurological disorders (mean age: 78.47 ±10.52 , min age: 56, max age: 96, 44% females).
T1 Weighted Images
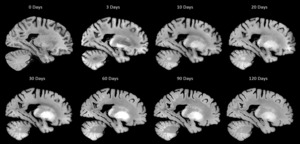
As the process of fixation with formalin significantly impacts T1 relaxation times and consequently the gray-to-white matter contrast of the tissue in T1 weighted (T1w) MRIs.5,7 Standard scans with Magnetization Prepared RApid Gradient Echo sequence (MPRAGE) were performed after a minimum of 4 months of fixation, to allow for the fixation process to complete for all brain regions, providing improved tissue contrast and signal to noise ratio (SNR). To verify the impact of fixation on different MRI contrasts, a subset of the specimens (N = 10) was also longitudinally scanned, from 0 to 120 days of fixation with 10% formalin. Figure 2 shows the impact of fixation on co-registered T1w MRIs of a DBCBB brain specimen that was scanned longitudinally from 0 days to four months of fixation. Note the changes in gray matter to white matter tissue contrast, noticeable at 3 days of fixation in the cerebellar and deep gray matter regions, expanding to the occipital lobe, and stabilizing in all brain regions after 90-120 days of fixation.
T2 Weighted Images
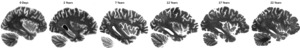
While T2 relaxation times tend to decrease over long periods (years) of fixation,8,36 the process of fixation does not significantly impact tissue contrast (i.e. gray to white matter or lesion to healthy tissue) in T2 weighted (T2w) MRIs,5,37 and intensity normalization can standardize the overall intensity range of T2w images with different fixation times. Figure 3 shows intensity-normalized (linear normalization) sagittal slices of T2w images acquired from six different DBCBB specimens that were fixed from 0 days to 22 years, showing similar relative gray-to-white matter tissue contrasts to those observed in vivo T2w images. Note the relatively stable tissue contrasts for the long-term fixed tissues, allowing for use of a consistent protocol across different fixation times.
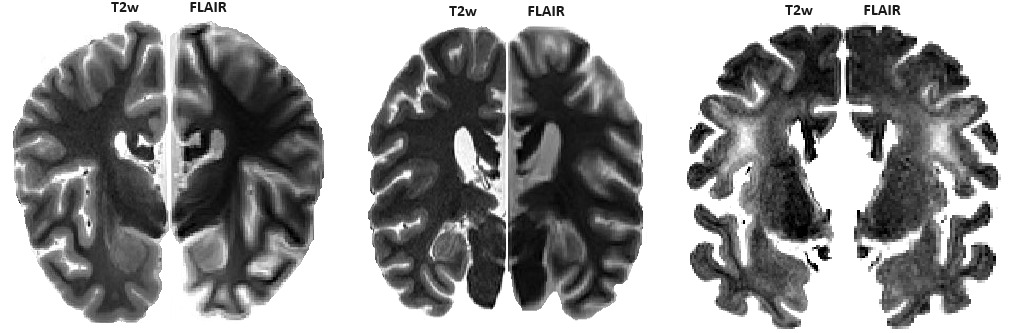
In vivo, Fluid-attenuated inversion recovery (FLAIR) images are usually acquired for the detection of white matter hyperintensities (WMHs), since nulling of the cerebrospinal fluid (CSF) allows for easier differentiation of the WMHs from the CSF.38 We did not include FLAIR images in our post-mortem protocol, as the fluid signal is no longer nulled for the formalin. As such, ex vivo FLAIR images do not provide additional information to the ex vivo T2w images, which can also be used to accurately detect and quantify WMHs both in vivo and ex vivo.39–41 While the parameters can potentially be adjusted to null the fluid signal, they would also impact the tissue contrast, resulting in additional variability in the acquired images. See Figure 4 for examples of T2w and FLAIR images in three DBCBB specimens. The FLAIR images are acquired with parameters consistent with those used in in vivo applications (TI= 2.5, TE=0.125, TR= 9, FA= 165). Our standard T2w images are shown as the hemispheres on the left, and the corresponding FLAIR slices are flipped and shown as the hemispheres on the right to enable comparison. Also note the WMHs that are clearly visible on the T2w images (specimen shown on the right), with a high correspondence to the FLAIR-based WMHs.
Diffusion Weighted Images
The process of fixation also significantly impacts the diffusion-weighted images (DWI), where T1, T2, and T2* relaxation times as well as diffusivity decrease in formalin-fixed tissues.23,36,42,43 Temperature is another factor that significantly impacts relaxation times and diffusivity in post-mortem tissues, with mean diffusivity (MD) values reportedly decreasing by ~30% when the brain temperature is decreased from body temperature (~37° C) to room temperatures (~20° C).44 As such, the impact of temperature and fixation on diffusion metrics must be modeled and adjusted for, to enable comparison with in vivo metrics.
The overnight acquisition protocols include five b-values: 1000, 2000, 3000, 4000, and 5000 s/mm2. To find the optimal parameters for the DWI acquisition in the standard protocol, multiple DWI sequences were acquired for a subset of the specimens with a range of directions (30, 64, and 95) and b values (1000, 2000, 3000, 4000, and 5000 s/mm2). Based on these experiments, compared to including all five b-values, the b-value combination of 2000 s/mm2 and 5000 s/mm2 (64 directions) provided the optimal results in terms of signal-to-noise ratio, consistency of FA and MD maps and tractography results, while keeping acquisition time feasible. Figure 5 shows an example of the calculated MD and FA maps for a DBCBB specimen with AD (fixation duration: 7 months), using all the acquired b values (the hemispheres on the right) as well as using just b = 2000 s/mm2 and b = 5000 s/mm2 (corresponding slices, flipped and shown as the hemisphere on the left). Performing probabilistic tractography on the preprocessed DWI volumes, we were able to identify the corticospinal tract in both acquisition protocols as shown in the right panel (Figure 5).
Quantitative Sequences
Three 3D gradient echo (GRE) sequences are acquired with different flip angles to calculate quantitative T1 maps.45,46 This variable flip angle (VFA) method with the addition of turbo-flash B1 map can be used to calculate T1 and T2* maps. Furthermore, the currently used sequences that allow for T1 mapping free of bias fields to minimize inhomogeneity in transmit B1 (known as MP2RAGE)45,46 did not provide sufficient contrast between gray and white matter on long term fixed brains. The 3D meGSE sequence acquires 32 TEs in 7:16 minutes and allows for fitting the multi-exponential model to generate T2 and MWF maps.33 As mentioned previously, since the process of fixation and to a lesser extent temperature impact T1, T2, and T2* relaxation times,36,42,44 the impact of temperature and fixation needs to be modeled and adjusted for, to enable comparison with in vivo quantitative metrics. Figure 6 shows the T1, T2, T2*, and MWF maps for one specimen. As expected and similar to previous reports,36,47,48 T1, T2, and T2* values are shorter compared to in vivo settings.
Quantitative Susceptibility Mapping
Another 3D multi-echo gradient echo sequence with 12 echoes is performed to calculate QSM maps. QSM maps can be used to determine the bulk magnetic susceptibility distribution of tissue, and are linked to iron concentrations in the brain. Note that the flip angle and number of echoes for this sequence are different from those of the VFA sequence, and are optimized for QSM.49 Figure 7 shows examples of the derived QSM maps for two DBCBB specimens. The ex vivo QSM maps show similar features to those observed in vivo as well as in other ex vivo studies,50 i.e. higher magnetic susceptibility in the subcortical gray matter regions which are known to be rich in iron.
Overnight 3T High-Resolution Scans
Figure 8 shows the co-registered standard resolution (coronal slice, shown as left hemisphere) and overnight high resolution (corresponding coronal slice, flipped and shown as right hemisphere) T1w (isotropic 0.7 mm3 versus 0.5 mm3) and T2w (isotropic 0.64 mm3 versus 0.5 mm3) images. Note that no preprocessing (i.e. denoising or intensity inhomogeneity correction) has been performed on any of the scans, to allow for a fair comparison. The overnight scans achieve higher signal-to-noise ratio and significantly greater anatomical details as they are scanned overnight with hour-long acquisitions, allowing for better differentiation of subcortical gray matter structures and gray-to-white matter surfaces. This is particularly noticeable in the area of the external and extreme capsule where the claustrum can be precisely delineated in the high-resolution images, but not in the standard resolution ones. The nuclei of the subthalamic area, as well as the hippocampal folding and subsegments are also regions where the high-resolution images remarkably benefit the precision of the structures thanks to the decreased partial volume at the perimeters of these intricate brain nuclei and cortex regions.
7T Imaging of Tissue Blocks
Figure 9 shows co-registered 3T and 7T images of the same DBCBB specimen for a tissue block containing the left hippocampus. Note the high gray-to-white matter contrast of both images as well as the added level of anatomical detail in the 7T images. Figure 10 shows the corresponding slices of T1w and T2w images of the same tissue block, as well as the derived T1 map, T2 map, and MWF results.
Discussion
In this work, we present a post-mortem MRI protocol that has been developed for the DBCBB specimens, allowing for data acquisition from a large number of brains without jeopardizing tissue quality for follow-up histology and immunohistochemistry assessments. While performing large-scale harmonized and standardized in vivo imaging is a possibility for cohort studies of disorders such as neurodegenerative dementias,51,52 this might not be the case for example for accidental deaths and/or suicide cases. Ex vivo MRI would therefore be an ideal method for obtaining anatomical and morphometric information of the intact brains in such cohorts before histology and immunohistochemistry assessments.
While post-mortem MRIs have been performed at smaller scales,5–7,16,23,53 large-scale high-resolution multi-modal post-mortem MRIs of intact brain specimens with a variety of neurodegenerative and mental health disorders has not been systematically performed. With our access to over 3000 post-mortem brain specimens at the DBCBB and the 3T and 7T imaging facilities at the Douglas CIC, we are able to perform ex vivo scans of hundreds of post-mortem brains each year, building an invaluable repository of consistently acquired post-mortem MRIs of neurodegenerative and mental health disorders. To facilitate translation to in vivo and clinical settings, we aimed to acquire sequences that were similar or comparable to their in vivo counterparts.
The acquired high-resolution isotropic T1w and T2w 3T images with high contrast for gray and white matter allow for accurate volumetric and morphometric assessments of brain structures and quantification of abnormalities such as WMHs and tumors, whereas the DWI and quantitative sequences enable assessment of tissue microstructure and biophysical properties, once the impact of temperature and fixation are appropriately adjusted for. While additional processing of the post-mortem tissue (e.g. soaking of the brains for improved diffusion, suspension in agar) might improve the quality of the post-mortem MRIs in certain cases, they also introduce additional artifacts (e.g. intensity gradients and lines caused by inconsistent penetration of fluid, additional air bubbles trapped in the sulci and ventricles).7 Furthermore, they might impact the quality of the tissue for downstream histology and immunohistochemistry assessments and were generally not feasible for the large-scale of our ex vivo program. Instead, we chose to acquire a large number of consistently processed and scanned post-mortem images with a large range of fixation durations (i.e. between 0 days to 22 years), allowing for future development of models that can be employed to adjust for the impact of fixation.23
Ex vivo DWI acquisition is particularly challenging due to a number of factors, including a decrease of mean diffusivity with a decrease in temperature,44 shortening of T2, and the consequent decrease in SNR.23 As such, ex vivo DWI acquisitions generally have poorer image quality and diffusion contrast compared to in vivo settings.5,23 While modifications to in vivo protocols combined with significantly longer acquisitions times (24 hours to 5 days) can lead to higher quality ex vivo DWI images,23 they were not feasible for our specific application. Instead, we acquired relatively lower resolution images (isotropic 2 mm3, 1.6 mm3, and 0.5 mm3 for the standard 3T, overnight 3T, and overnight 7T protocols, respectively). While the resulting MD and FA values were lower than their in vivo counterparts, we were able to retrieve the expected pattern in gray and white matter regions in MD, and the overall FA patterns within the white matter (Figure 5, left panel). Since the corpus callosum is cut by the sagittal split of the hemispheres, we used probabilistic tractography to identify the corticospinal tract as a quality control step (Figure 5, right panel).
The main challenge in ex vivo acquisition of the quantitative sequences pertained to the presence of small air bubbles in the ventricles and sulci that can lead to image artifacts, particularly in acquisitions with higher echo times and in the fresh tissue, where there was a greater likelihood for presence of air. In cases of prolonged fixation, air bubbles were naturally released in time, and we generally did not find air artifacts in the resulting images. In cases with shorter fixation periods (e.g. the longitudinally scanned specimens that had to be scanned from day 0), we slowly rotated the containers to release the air trapped in the sulci and ventricles. While there is no gold standard method to remove air bubbles from the tissue, we found slow rotation over time to be the most effective method as more aggressive techniques risk damaging the tissue and creating additional deformations, in particular in cases of specimens with extensive atrophy (e.g. neurodegenerative disorders). For some cases, using an orbital shaker at slow speed for 5-10 minutes or degassing using a vacuum chamber also helps remove air bubbles from the tissue. We further avoided the transfer of tissue across containers (except for the first three weeks of fixation, see the Methods section) to prevent the addition of further air bubbles.
The proposed ex vivo protocol is not without limitations, most of which pertain to choices that were made to maintain its feasibility for large scale acquisitions in the context of tissue provided by brain banks. The specimens are scanned in Formalin and not Fomblin (or similar solutions such as Christolube) which provide a zero signal background.23,37,54 This decision was made for the following reasons: i) transfer between the solutions exposes the tissue to air that gets trapped in the ventricles and sulci, leading to artifacts in the acquired images particularly for brains with extensive atrophy, ii) Fomblin and Christolube are high density fluids that push the tissue to the edge and lid of the container, causing substantial deformation in the tissue, iii) due to their oil-like properties, residual Fomblin and Christolube are difficult to fully remove from the surface of the tissue, necessitating manual embedding for subsequent tissue processing and histological staining,23,55 and iv) both solutions are relatively expensive (>$500 Canadian dollars per liter), and the quantities necessary for large scale projects can become financially prohibitive.55 Similarly, to maintain feasibility with respect to acquisition times, the proposed protocol does not include ultra high-resolution images and sampling schemes such as those performed in studies that acquire ex vivo MRIs dedicated to a specific methodology for limited numbers of samples or with custom designed MRI coils.23,56,57
The developed ex vivo imaging protocol and the acquired images are part of an ex vivo imaging program that was initiated by the corresponding authors (MD and YZ). Future plans involve acquisition of post-mortem images for all existing and incoming brain specimens at the DBCBB. Anonymized images as well as the preprocessed outputs (e.g. quantitative maps, DWI metrics) and all other information (e.g. demographics, medical history, and detailed neuropathology assessments) can be directly requested from the DBCBB. High resolution images are currently acquired on a research project specific basis, and interested researchers can contact the corresponding authors to request higher resolution images or additional protocols and sequences to be performed for specific specimens provided they cover the acquisition costs.
In conclusion, high-resolution post-mortem imaging can provide invaluable information on the intact brain before sectioning of the tissue for histology. The ex vivo images can complement information from in vivo imaging where in vivo imaging is possible, and provide novel information in the case of disorders where in vivo imaging might be challenging. Finally, linked to histology and immunohistochemistry assessments of the same tissue, ex vivo images can be used to develop MRI biomarkers that can quantify various post-mortem pathologies with greater sensitivity and specificity, with potential for future in vivo implementation and translation into clinical applications to detect and monitor these pathologies in clinical trials and patient care. A post-mortem imaging protocol that can be feasibly implemented for large-scale scanning of brain bank specimens is therefore of great value for researchers.
Acknowledgements
Dadar reports receiving research funding from the Healthy Brains for Healthy Lives (HBHL), the Quebec BioImaging Network (QBIN), the Natural Sciences and Engineering Research Council of Canada (NSERC), and Brain Canada. Zeighami reports research funds from the HBHL and NSERC. Maranzano reports receiving funding from the QBIN and the NSERC. The Douglas-Bell Canada Brain Bank is supported in part by platform support grants from the Réseau Québécois sur le Suicide, les Troubles de l’Humeur et les Troubles Associés (FRQ-S), Healthy Brains for Healthy Lives (CFREF), and Brain Canada. We would also like to thank Danae Lussier-Dumouchel, Elena Drobotea, Mila Urosevic, and Alyssa Guerra for their help in MRI data acquisition and preparation of post-mortem specimens.
Conflicts of Interest
The authors have no conflicts of interest to declare.



_and_fractional_anisotropy_(fa)_maps_in_white_matter_for_.png)
_maps_for_one_dbcbb_specimen_wer.png)
_images_for_two_dbcbb_specimens_estimated_based_o.png)


_maps_for_one_dbcbb_spe.png)



_and_fractional_anisotropy_(fa)_maps_in_white_matter_for_.png)
_maps_for_one_dbcbb_specimen_wer.png)
_images_for_two_dbcbb_specimens_estimated_based_o.png)


_maps_for_one_dbcbb_spe.png)