Precision TMS
Neuromodulation is the use of invasive or non-invasive devices to excite or inhibit neuronal activity in order to treat brain disorders and enhance cognitive functions. It represents an important scientific frontier in brain health, brain-machine interfaces, and brain-computer integration, with significant applications in fields such as healthcare, education, and military. Taking transcranial magnetic stimulation (TMS) as an example, it has been widely used in cognitive research and in the treatment and rehabilitation of various brain disorders such as depression, pain, addiction, obsessive-compulsive disorder, movement disorders, dementia, epilepsy, and stroke. Compared to medication, neuromodulation techniques offer advantages such as safety, minimal side effects, and long-lasting effects. They have become the future trend in the intervention of intractable brain disorders. However, existing technologies cannot achieve precise modulation tailored to individuals, resulting in generally low clinical efficacy rates in various brain disorder treatments. For example, the remission rate of TMS in treating depression is only about one-third, significantly limiting its clinical application.
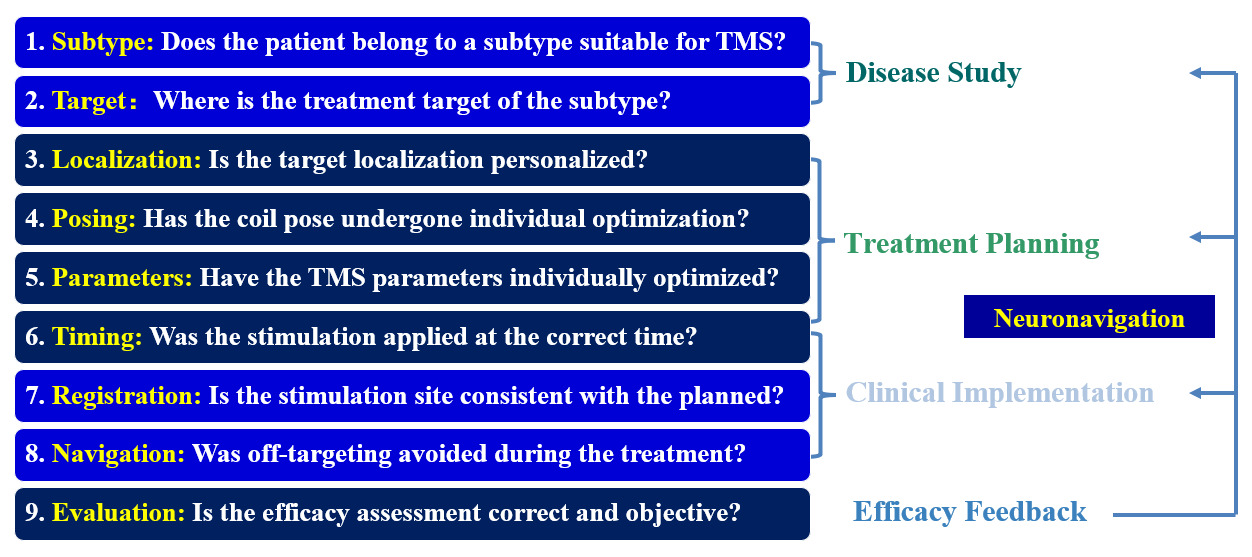
Patient-specific, precise TMS is the key to revolutionizing this neuromodulation technique, enabling efficiency improvements in clinical applications and ensuring reproducibility in basic research.1 As illustrated in Figure 1, Precision TMS consists of 4 major components: Understanding of the disease, formulation of individualized treatment plans, precise implementation of treatment plans, and objective and accurate evaluation of therapeutic effects. The last step provides feedback information, continuously strengthens learning in the previous steps, and ultimately achieves the best outcome.
Precise TMS begins with comprehensive characterization of the underlying neuropathology. This involves multimodal assessment, including clinical symptoms, neuroimaging (fMRI, DTI), neurophysiological markers (EEG, EMG), and genetic profiling when applicable.2 Such thorough evaluation identifies target neural circuits and their functional abnormalities, forming the scientific basis for personalized intervention strategies. Current advances in connectome mapping and computational modeling now allow unprecedented resolution in understanding disease-specific network dysfunction.
Building on disease insights, clinicians develop tailored protocols considering individual neuroanatomy (cortical geometry, gyral patterns), functional connectivity, and treatment history. Modern neuronavigation systems integrate this data with brain atlases to calculate optimal stimulation parameters (target location, coil orientation, stimulation intensity, etc.). Emerging AI-assisted platforms3,4 can now simulate predicted electric field distributions, enabling data-driven optimization of treatment parameters before actual delivery.
Accurate plan implementation requires advanced neuronavigation with real-time tracking (typically <2mm error). Robotic TMS systems enhance precision through automated coil positioning with force control, while machine vision maintains targeting during head movement. Closed-loop systems incorporating concurrent EEG5,6 / fNIRS7–9 monitoring enable dynamic parameter adjustment, ensuring consistent neuromodulation of intended circuits despite biological variability.
Quantitative assessment combines clinical scales with neurophysiological (TMS-EMG, ERP) and imaging biomarkers to measure treatment efficacy. These metrics feed into adaptive learning algorithms that refine future interventions. Recent developments include digital phenotyping through wearable devices and cloud-based analytics, creating continuous feedback loops that progressively optimize the entire precision TMS workflow for both individual patients and population-level insights.
Robot-assisted neuroimage-guided neuromodulation represents a transformative advancement in precision TMS, integrating cutting-edge engineering with neuroscience to achieve unprecedented accuracy in brain stimulation. By leveraging individualized brain atlases and multimodal neuroimaging data, this approach enables meticulous treatment planning and objective outcome evaluation. The robotic system serves as the critical enabling technology, translating these sophisticated plans into precise, real-world interventions with spatial fidelity and real-time motion compensation. This synergy of computational neuroanatomy and mechatronic precision not only enhances the reliability of neuromodulation delivery but also establishes a foundation for closed-loop, adaptive TMS. The following discussion will explore how robotic automation is redefining the standards of accuracy, reproducibility, and personalization in neuromodulation, while addressing both the technical innovations and clinical validations that underscore its growing adoption.
Neuromodulation Robot
A comparative study demonstrated that manual coil positioning using basic mechanical holders introduces significant deviations in the induced electric field distribution, while robotic navigation systems maintain continuous targeting accuracy within clinically acceptable tolerances throughout the entire treatment session, ensuring consistent electric field delivery to the intended cortical target.10 Current robotic systems in clinical use, such as the invasive neurosurgical platforms ROSA One,11 Remebot,12 and Sinovation,13 along with the non-invasive TMS Cobot system by Axilum Robotics14 and Vmove from ANT Neuro,15 are fundamentally neuronavigation robots rather than complete neuromodulation solutions. These systems excel in localization precision and have demonstrated clinical utility in electrode placement and coil positioning. However, they remain limited to the navigation component of neuromodulation, lacking two critical features that would qualify them as true neuromodulation robots: (1) intelligent software for formulating individualized treatment plans based on multi-modal neuroimaging and physiological data, and (2) integrated hardware combining navigation with actual modulation devices (e.g., built-in TMS components). This architectural limitation persists because current systems typically interface with separate TMS devices rather than incorporating them as unified systems.
The next generation of TMS robots must bridge this gap by developing personalized treatment planning algorithms, accurate spatial registration and active motion compensation methods, closed-loop systems that incorporate real-time neurophysiological monitoring, and fully integrated stimulation hardware to achieve true precision TMS.
Personalized Target
Personalized targeting in TMS is crucial because individual neuroanatomical and functional variability significantly influences stimulation outcomes. Studies have demonstrated that standard scalp-based targeting methods, such as the “5 cm rule” or the “Beam F3” approach, often result in inaccurate coil placement due to differences in head size, cortical folding, and functional neuroanatomy.16,17 For example, MRI-based analyses reveal that the dorsolateral prefrontal cortex (DLPFC), a common TMS target for depression, can vary in location by up to 2–3 cm across individuals.18 Without accounting for these variations, stimulation may miss the intended cortical region, reducing treatment efficacy.
The use of standardized brain atlases for TMS targeting fails to account for individual neuroanatomical variations, leading to suboptimal stimulation accuracy. Brain atlas individualization addresses this limitation by warping population-based atlases to subject-specific anatomy using high-resolution structural MRI, preserving unique cortical folding patterns and functional boundaries.19 The Brainnetome Atlas20 has been successfully used to enable precise, individualized TMS targeting across multiple neurological and psychiatric disorders.21 By warping the Brainnetome Atlas to patient-specific MRI data, the authors developed a standardized yet personalized approach to identify cortical targets, addressing the limitations of traditional landmark-based methods. The pipeline was successfully applied to several clinical conditions, including major depressive disorder and schizophrenia (targeting the dorsolateral prefrontal cortex, DLPFC), Tourette syndrome (targeting the supplementary motor area, SMA), and tinnitus (targeting the auditory cortex). Additionally, the method proved useful for neurosurgical language mapping (Wernicke’s area) and motor cortex localization. The integration of connectivity-based parcellation with individual neuroanatomy represents a meaningful advance in precision TMS. The accuracy of atlas warping significantly impacts individualized targeting, necessitating sophisticated algorithms like the GNN-based method22 to ensure precise alignment. This advancement represents a paradigm shift from traditional gross anatomical targeting to true precision neuromodulation that accounts for each patient’s unique brain architecture.
Beyond anatomical differences, functional connectivity also varies between patients. Resting-state fMRI studies show that the same anatomical region (e.g., DLPFC) may have different network interactions across individuals.23 Personalized targeting based on functional connectivity maps, rather than standardized coordinates, has been shown to improve antidepressant response rates.24 For example, targeting the DLPFC subregion most negatively correlated with the subgenual cingulate (a depression-related hub) leads to better clinical outcomes compared to non-personalized approaches.25 Personalized targeting was critical in the success of Stanford Neuromodulation Therapy (SNT).26 Using MRI-guided neuronavigation, researchers precisely targeted each patient’s left DLPFC based on individual anatomy and functional connectivity, avoiding the variability of scalp-based methods. This precision contributed to SNT’s remarkable 79% remission rate, demonstrating how personalized targeting maximizes TMS efficacy in depression treatment.
Additionally, electric field (E-field) modeling reveals that individual differences in skull thickness, cerebrospinal fluid distribution, and cortical folding alter the strength and spread of TMS-induced currents.27 Studies using computational modeling demonstrate that fixed-intensity TMS can result in understimulation or overstimulation depending on scalp-to-cortex distance.28 Personalized E-field-guided dosing ensures that sufficient current reaches the target while minimizing side effects.29
While personalized targeting through neuroimaging and atlas individualization has revolutionized TMS planning by accounting for anatomical and functional variability, the full therapeutic potential can only be realized through equally precise coil placement that maintains optimal positioning and orientation relative to the individualized target throughout stimulation.
Optimal Coil Placement
Following target identification, coil positioning and orientation require patient-specific optimization to account for individual neuroanatomical variations. Even when stimulating the same cortical target, differences in coil placement can alter the induced electric field in both magnitude and spatial distribution, potentially compromising treatment efficacy. The integration of electric field (E-field) simulation into TMS treatment planning represents a transformative advancement in precision neuromodulation. Modern computational approaches leverage patient-specific anatomical data from MRI scans to model the distribution and magnitude of induced electric fields throughout cortical and subcortical structures.30,31 These simulations solve the electromagnetic field equations while accounting for individual variations in skull geometry, cerebrospinal fluid distribution, and gray/white matter boundaries, providing crucial insights that guide optimal coil placement.32,33
Sophisticated finite element method (FEM) modeling has revealed substantial interindividual variability in electric field distributions, with studies demonstrating 30~40% differences in peak field strength between subjects receiving identical stimulation parameters. This variability stems from anatomical differences in cortical folding patterns, tissue conductivity profiles, and skull morphology. Advanced simulation platforms now incorporate anisotropic conductivity models that differentiate between white matter fiber directions, significantly improving the accuracy of predicted field patterns compared to earlier isotropic approximations.
For clinical implementation, these simulations inform three critical positioning parameters: (1) the precise cortical target location that maximizes field strength in the desired neural circuits while minimizing off-target excitation, (2) the optimal coil tilt angle that accounts for local gyral geometry to achieve effective neuronal depolarization, and (3) the stimulation intensity required to reach therapeutic thresholds in the target tissue. Contemporary neuronavigation systems should integrate these simulation results directly into their planning interfaces, allowing clinicians to visualize predicted field distributions superimposed on the patient’s anatomy before treatment.
While finite element method (FEM)-based simulations are often computationally intensive and time-consuming, recent advances have enabled real-time electric field modeling capabilities.29,34 Furthermore, machine learning algorithms trained on large datasets of simulated fields now enable rapid approximation of optimal coil placement without requiring computationally intensive simulations for every patient, making personalized field modeling more clinically practical.35,36
An alternative to computationally intensive FEM simulations is to construct a coil pose atlas as a group-level prior for rapid personalized coil placement optimization, leveraging fast E-field approximations. The Stimulation Effects Mapping (SEM) framework has been proposed to precompute optimal coil placements (position and orientation) across 18 subjects in a standardized head-anatomy-based polar coordinate (HAC) system, integrating intensity and focality metrics of the E-field.37 By mapping these priors to individual anatomy without requiring subject-specific segmentation or meshing, SEM achieves consistent targeting across 74 cortical regions defined in the Brainnetome atlas (Dice coefficient: 0.806 ± 0.144) and outperforms auxiliary dipole methods in E-field strength within target ROIs. The framework provides coordinates in both MNI and custom HAC systems, enabling clinical translation while reducing computational costs from hours to real-time application.
At the core of the transformation from neuronavigation robots to neuromodulation robots is the integration of sophisticated treatment planning algorithms that analyze each patient’s unique neuroanatomy and functional connectivity.38,39 By processing multimodal neuroimaging data, these systems can predict optimal stimulation targets and coil placements before treatment begins. This represents a fundamental shift from current approaches that primarily focus on accurately reproducing predetermined targets without considering individual variations in brain structure and function. To fully realize this precision, neuromodulation robots must incorporate advanced neuronavigation capabilities, including accurate spatial registration to align imaging and physical spaces, dynamic head motion compensation to maintain targeting accuracy during sessions, and off-target stimulation recording to control for confounding effects in treatment optimization.
Neuronavigation Robot
The effectiveness of neuronavigation robot hinges on the seamless integration of accurate robotic manipulation, real-time motion tracking, and adaptive control systems, ensuring precise execution of personalized treatment plans. As illustrated in Figure 2, the RC-M2N2-Duet (REALControl, China) neuronavigation robot consists of two core components: a robotic arm and a camera system.40 The robotic arm is responsible for precise coil positioning and orientation adjustments, while the camera system tracks head movements and ensures real-time spatial registration between the patient’s anatomy and the planned stimulation targets. Together, these systems enable dynamic compensation for head motion and maintain targeting accuracy throughout the treatment session. The integration of these components is critical for translating personalized treatment plans into accurate and consistent stimulation delivery.
Robotic arms used in neuronavigation systems come in various forms, including Cartesian gantries, SCARA robots, and articulated collaborative robots (cobots). Cartesian systems offer linear precision but lack flexibility for complex trajectories, while SCARA robots provide speed but limited dexterity. Articulated cobots, such as those with six or seven degrees of freedom, emerge as the optimal choice due to their adaptability, safety features, and ability to navigate around the patient’s head while maintaining precise coil placement. Their collaborative design allows for seamless interaction with clinicians, making them ideal for clinical environments where safety, precision and workflow efficiency are paramount.
The camera system in neuronavigation robots can employ infrared, structured light, or visible light technologies. Infrared cameras offer high tracking accuracy but require reflective markers and specialized hardware, increasing costs and complexity. Structured light systems provide detailed 3D surface mapping but are sensitive to ambient light and involve higher expenses. In contrast, visible light cameras present a cost-effective and versatile solution. Beyond motion tracking, they enable video-based adverse event detection (e.g., seizures or discomfort) and micro-facial expression analysis to gauge patient responses in real time. Additionally, their compatibility with telemedicine platforms opens possibilities for remote therapy supervision and AI-assisted treatment optimization. With rapid advances in computer vision, visible light systems are poised to become the preferred choice, balancing affordability, functionality, and scalability for next-generation neuromodulation robots.
The robotic control systems incorporate sophisticated kinetic and dynamic modeling to maintain optimal coil positioning throughout treatment. By analyzing head movement patterns and integrating data from multiple force and position sensors, they can adjust coil placement in real-time with sub-millimeter accuracy. Advanced contact detection systems monitor impedance changes at the coil-scalp interface, automatically adjusting contact pressure to ensure consistent coupling while maintaining patient comfort. This dynamic control is particularly valuable for extended treatment sessions and for patients with movement disorders who may have difficulty remaining still.
Software and Hardware Integration
Unlike current systems that require separate navigation and stimulation components, software and hardware delivered from different companies, true neuromodulation robots feature fully integrated TMS hardware with synchronized robotic control.
By combining navigation and stimulation components into a unified platform, these systems achieve precise microsecond-level synchronization between coil positioning and pulse delivery. This tight integration enables dynamic adjustments that maintain targeting accuracy despite patient movement while simultaneously allowing closed-loop adaptation of stimulation parameters based on real-time neurophysiological feedback from EEG or other monitoring modalities. Closed-loop integration of tractography32 and fMRI41 allows dynamic refinement of targets based on structural and functional feedback, transforming robotic TMS into a tool for causal brain mapping.
Beyond basic targeting improvements, this robotic integration fundamentally enhances treatment reproducibility and protocol standardization. The automated recording of exact coil coordinates paired with stimulation parameters for each pulse creates an unprecedented level of quality control and data traceability. The system’s capabilities extend to automating essential clinical procedures including motor hotspot localization and resting motor threshold determination, thereby ensuring consistent personalized dosing while reducing operator-dependent variability.
These technological advances unlock new possibilities for both clinical applications and neuroscience research. The precision and reliability of integrated robotic systems facilitate complex stimulation paradigms such as multi-target simultaneous and/or sequential stimulation and network-level neuromodulation protocols. The use of multiple robotic arms, each holding a separate coil, further enhances feasibility for such advanced applications. Furthermore, the inherent programmability and remote operation capabilities of these systems open doors to novel applications ranging from telemedicine solutions, e.g. in cases of rural areas and space missions, to adaptive therapies for neurological disorders. This convergence of robotics and neuromodulation represents not just an incremental improvement, but rather a fundamental shift toward more reproducible, automated, and scientifically rigorous brain stimulation methodologies.
Challenges and Potential Solutions
The development of neuromodulation robots faces several key challenges across algorithm design, hardware implementation, and system integration. On the algorithmic front, brain atlas-based treatment planning must evolve to better account for individual neuroanatomical variations while maintaining computational efficiency. Advanced space registration techniques are needed to seamlessly map imaging data to physical space, ensuring accurate targeting despite anatomical diversity. Robotic control algorithms must achieve sub-millimeter precision in coil placement while maintaining safety during dynamic adjustments. Visualization and simulation tools require further refinement to provide intuitive, real-time feedback during procedures. Cloud computing platforms could potentially address the computational demands of personalized targeting while enabling collaborative protocol development and data sharing.
Hardware limitations present another major hurdle. Robotic arms must balance precision with practicality, moving toward compact and desktop designs. Camera systems need enhanced robustness to perform reliably in suboptimal clinical environments, with emerging solutions potentially leveraging smartphone camera technology for cost-effective motion tracking. Stimulator redesigns are equally critical, particularly for developing portable systems that maintain therapeutic efficacy while expanding treatment settings beyond traditional clinics. A wearable TMS device weighing under 3 kg42 has been developed, matching the performance of conventional systems while enabling clinical accessibility and home-based interventions.
System integration remains the most complex challenge, requiring tight software-hardware co-optimization to achieve seamless operation. Data standardization efforts, such as the BIDS Extension Proposals for NeuroNav and NIBS, are laying crucial groundwork for reproducible research by establishing unified protocols for file naming and storage. Video-based analysis systems are being developed to detect adverse events in real time while providing valuable data for protocol optimization. Looking ahead, the integration of large AI models could revolutionize treatment automation, much like autonomous vehicle systems, future neuromodulation robots may eventually plan and execute complete treatment regimens automatically upon receiving patient neuroimaging and clinical data. This end-to-end automation paradigm represents both the greatest challenge and most promising direction for next-generation neuromodulation systems.
Harmonizing protocols across labs requires collaborative frameworks for data and parameter sharing. Initiatives like the BIDS Extension for NeuroNav (https://github.com/bids-standard/bids-specification/issues/1409, https://github.com/bids-standard/bids-specification/issues/1110) establish standardized file formats for neuronavigation data, while cloud-based platforms (e.g., The Virtual Brain) enable replication of stimulation paradigms. Future efforts should prioritize interoperable robotic systems that adhere to these standards, ensuring reproducibility in multicenter trials.
Conclusion
Neuromodulation robots represent a significant evolution in TMS therapy, integrating intelligent treatment planning, real-time adaptation, and closed-loop monitoring to achieve personalized stimulation. These systems address key limitations of conventional approaches by combining precise targeting with dynamic adjustment capabilities, leading to improved treatment consistency and clinical outcomes. Current evidence demonstrates their superiority in delivering accurate electric fields to target regions compared to traditional methods.
The integration of robotic neuromodulation with large-scale brain mapping initiatives (e.g., Human Connectome Project, EBRAINS) offers a transformative opportunity to bridge correlative imaging findings with causal network manipulation. By aligning precision TMS protocols with the mission of OHBM (https://www.humanbrainmapping.org/) to standardize brain data, these systems can empirically test and refine connectome-based hypotheses, advancing both therapeutic applications and fundamental neuroscience.
The technology’s potential extends across research and clinical applications, enabled by advances in computational modeling, robotic control, and compact hardware design. By maintaining treatment precision while improving accessibility, neuromodulation robots may facilitate broader implementation of personalized TMS protocols. Future developments in AI integration and system miniaturization will likely expand their therapeutic scope, particularly for complex neurological conditions requiring network-level modulation. This technological progression marks a critical step toward more effective and standardized neuromodulation therapies.
Acknowledgments
This work was partially supported by the Science and Technology Innovation 2030 - Brain Science and Brain-Inspired Intelligence Project of China (Grant No. 2021ZD0200200) and by the National Natural Science Foundation of China (Grant Nos. 82151307, 62327805, and 62336007).
Conflicts of Interest
The authors declare that they have no competing interests.