Introduction
Perinatal stroke (PS) occurs in 1:1000 births and can result in motor, cognitive, and visual impairments that can affect independence throughout the lifespan.1,2 PS can be classified as a group of six specific cerebrovascular diseases defined by mechanism and timing of injury.1 PS occurs between 20 weeks of fetal life and 28 days of postnatal life, although roughly half of cases are not recognized until they present as motor impairment later in infancy with the remote stroke confirmed by imaging and labelled presumed perinatal stroke. The two most common forms of PS are arterial ischemic stroke (AIS), which occurs most often within the middle cerebral artery and results in large lesions near term, while periventricular venous infarction (PVI) results in smaller lesions and occurs in the midgestational stage.3 Both stroke types can inflict discrete injuries to posterior visual pathways between the lateral geniculate nucleus (LGN) and visual cortex.
As the leading cause of hemiparetic cerebral palsy (CP), the primary focus of PS research to date has been on motor impairments, where an improved understanding of developmental motor plasticity in both the lesioned and non-lesioned hemisphere has informed new clinical interventions.4 In contrast, few studies have aimed to explore the mechanisms of visual impairments that complicate PS. Research shows that asymmetry and microstructural differences of the optic radiations may be associated with visual outcomes after AIS when assessed at 3 months of age.5 Similar microstructural differences in the lesioned hemisphere have been described in children with periventricular and cortico-subcortical lesions with no clear differences between groups.6 There exists increasingly robust evidence of PS-induced alterations in the development of the non-lesioned hemisphere,7–10 yet this has not been examined in the visual system.
Cerebral visual impairment (CVI) refers to visual and perceptual deficits resulting from damage to the post-chiasmic pathways of the visual system, including the optic tract and optic radiations. It has been estimated that up to 70% children with cerebral palsy have CVI,11 and while PS is the leading cause of hemiparetic cerebral palsy, we currently do not know how many children with PS are experiencing CVI. CVI can negatively impact how children interpret visual information and engage with their environment, consequently affecting typical development in activities such as ambulation,12 social communication,13,14 and school performance,15 where even mild visual impairment is associated with decreased socioeconomic status in adulthood.16 Understanding the structural development of the visual pathways can support the advancement of diagnostic and prognostic tools for visual impairment in children with early brain injury and contribute to development of therapies to reduce functional impairment.
Here, using diffusion weighted imaging and tractography, we aimed to reconstruct the tracts of the afferent visual system in both hemispheres of children with PS and compare them between stroke subgroups and to typically developing controls. We hypothesized that structural development of the afferent visual pathways would be altered bilaterally in children with AIS.
Methods
Participants
One-hundred and one children aged 6-18 years with PS were recruited from the Alberta Perinatal Stroke Project,17 a population-based cohort: N=29 AIS (mean age 10.0 [SD 2.9], 12 males), N=29 PVI (mean age 10.2 [SD 3.1], 20 males), N=43 TDC (mean age 11.3 [SD 3.5], 20 males). Stroke group participants (AIS and PVI) were consented as part of a larger study, Stimulation for Perinatal Stroke Optimizing Recovery Trajectory (the SPORT trial)(https://www.clinicaltrials.gov/) and the cross-sectional baseline imaging is used in the current study. Inclusion criteria for the perinatal stroke group were: 1. Age 6–18 years with term birth (>36 weeks), 2. clinical and MRI-confirmed perinatal ischemic stroke (AIS, PVI), 3. symptomatic hemiparetic CP, 4. informed consent/assent. Exclusion criteria included other neurological disorders not related to perinatal stroke, diffuse or multifocal stroke, severe hemiparesis, severe spasticity (Modified Ashworth Scale >3), severe delay or inability to comply with protocol, unstable epilepsy, any MRI contraindication. Similarly aged typically developing controls were recruited via a community healthy controls recruitment database (HICCUP, www.hiccupkids.ca). Inclusion criteria for typically developing children were 1. Term birth, 2. No MRI contraindications, 3. Right-handed. Parental written informed consent and participant assent were acquired. This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary.
Neuroimaging acquisition
Images were acquired using a 3T GE MR750w scanner, and a 32-channel head coil, at the Alberta Children’s Hospital Diagnostic Imaging Suite in Calgary, Alberta, Canada. Single-shell diffusion images (60 directions, b=2000 s/mm2, 5 b0 volumes, 2.5mm isotropic voxels, repetition time (TR)/echo time (TE)=15s/87.5ms, duration=16:30 mins) were acquired in the axial plane. T1-weighted anatomical (166 slices, 1mm isotropic voxels, TR/TE=8.5/3.2ms, duration=4:53 minutes) sequences were also obtained in the axial plane.
Image processing
Diffusion image pre-processing was completed using MRtrix3, SPM, and Synb0.18–20 Standard diffusion data pre-processing steps were followed including denoising, Gibbs ringing removal, motion and eddy current correction. Synbo-Disco was used to create a synthetic undistorted b=0 image to enable echo planar image distortion correction. Anatomical segmentations were performed using SPM12.18 Resulting grey matter, white matter, and cerebrospinal fluid masks were combined to create a brain mask. In MRtrix3, fibre orientation distribution (FOD) maps were calculated using the constrained spherical deconvolution model. Subsequently, three-tissue response estimation was completed and used for 3-tissue CSD modelling.21 Final steps included bias field correction and multi-tissue informed log-domain intensity normalisation.22–24
Tractography
Probabilistic white matter tractography was completed in MRtrix320 to reconstruct the visual pathways in each hemisphere. Two regions of interest (ROI) were defined on coronal slices in each hemisphere [Figure 1, right]. The optic chiasm ROI was drawn on a coronal slice just posterior to the crossing fibres (red on the colour map) of the optic chiasm. The posterior thalamic radiation ROI was drawn on the coronal slice anterior to the splenium of the corpus callosum.25 Tracts in each hemisphere were reconstructed using the tckgen command and the iFOD2 probabilistic algorithm (maximum 5000 streamlines, maximum angle 450, stepsize 1.25mm). Participants whose tracts could not be reconstructed within these parameters were excluded. After reconstruction, spurious streamlines and those that were not anatomically plausible as visual tracts were removed using exclude ROIs. Spurious fibres removed included those belonging to the fornix, uncinate fasciculus, and splenium of the corpus callosum. Binary masks were generated from the final tracts and whole-tract mean fractional anisotropy (FA) and mean (MD) diffusivity were extracted in each hemisphere separately.
Along the tract metrics
To more precisely localise regions where white matter microstructure might be affected by lesion damage compared to TDCs, along-tract data extraction was performed. The tckresample function of MRtrix3 was used to identify and sample 11 different locations along the optic tract, LGN, and optic radiations (Figure 5A). First, the reconstructed visual tracts and the ROIs from the tractography procedure were overlaid on the colourmap, and a third ROI was added at the LGN. Coordinates were then extracted corresponding to the ROI placements in each hemisphere of each participant to define an arc projecting between the ROIs. For each of the equidistant 11 subsegments of the tract, FA and MD were extracted.
Assessing lesion location and size
Using T1-weighted anatomical images, lesions were classified by a pediatric neurologist (AK) into PVI and AIS subtypes. AIS lesions were further classified into proximal M1 (n=19), distal M1 (n=9) and lenticulostriate (n=1) vascular territories. To quantify lesion size, lesion masks were created in MRIcron using the 3D paint tool26 based on T1-weighted image intensity (difference at edge - 8) and consensus was achieved for accuracy of lesion demarcation by a secondary reviewer (HC). As PVI lesions often appear as enlarged ventricles, ventricular volume was calculated for both right and left hemispheres using the same procedure as above, and the ventricle volume of the non-lesioned hemisphere was subtracted from the lesioned hemisphere. Lesion volume was calculated from the binary lesion masks using FSL via fslmaths.27 To identify which along-tract segments were in close proximity to stroke lesions for the AIS group, tracts were overlaid on the coregistered image for each participant and each tract subsegment was coded as to whether it was colocalized with the lesion (1) or not (0).
Statistical Analysis
Statistical analyses were completed using Jamovi Version 2.2.5.0 and verified using R.28,29 Analysis of sex between groups was assessed using a chi-squared test of independence, analysis of age was completed using a one-way analysis of variance (ANOVA). For all statistical analyses, the non-dominant (right) hemispheres in right-handed controls were compared to the lesioned hemispheres of the PS groups. Data normality was assessed using the Shapiro-Wilk test. Between-group differences of FA and MD in the lesioned and non-lesioned hemispheres, and differences in the AIS group based on lesion location, were assessed using one-way Analysis of Covariance (ANCOVA) adjusted for age, or Kruskal-Wallis one-way ANOVA. Subsequent pairwise comparisons were assessed using either Tukey post-hoc tests, or Dwass-Steel-Critchlow-Fligner pairwise contrasts corrected for multiple comparisons (Bonferroni). Between-hemisphere differences were assessed using paired Student’s t-tests (or Mann-Whitney). Along the tract differences were examined using paired and independent T-tests. In the case of normal distribution, a Student’s t-test was used, and Mann-Whitney was utilized when the sample did not meet the assumption of normality. Effect sizes calculated using Cohen’s d or Rank biserial correlation based on normality.
Results
Seven participants were excluded from the AIS group as visual tracts could not be reconstructed in the lesioned hemisphere. All seven of these children had lesions affecting the proximal M1 artery. In total, 94 participants were included in the analysis (N=22 AIS, N=29 PVI, N=43 TDC). There were no significant sex (χ²1 =0.18, p = 0.67) or age (F~(2, 91)~= 2.422, p=0.09) differences between groups.
Demographics
Participant group differences
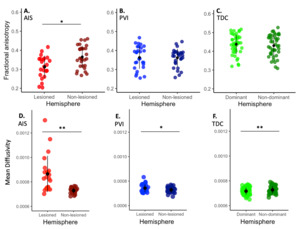
Tractography of the visual tracts was completed successfully for 22 children with AIS, 29 children with PVI and 43 controls with exemplars illustrated in Figure 2. Compared with TDCs, children with AIS had significantly lower FA (F2,91=30.26, p<0.001), and higher MD (χ²2 =35.6, p<0.001) in the lesioned hemisphere, along with lower FA (F3,90=27.41, p<0.001) and no difference in MD (F2,91=0.05, p=1.000) in the non-lesioned hemisphere (Figure 3). Comparing AIS to PVI, children with AIS had lower FA (F2,91=30.26, p=0.011) and higher MD (χ²2 =35.6, p<0.001) in the lesioned hemisphere, with no differences in the non-lesioned hemisphere (FA = (F3,90=27.41, p=1.000), MD = (F2,91=0.05, p=1.000)). Comparing PVI to TDCs, FA was lower in both the lesioned (F2,91=30.26, p<0.001) and non-lesioned (F3,90=27.41, p<0.001) hemispheres (t90=4.3, p<0.001), MD was higher in the lesioned hemisphere (χ²2 =35.6, p=0.007) with no difference in the non-lesioned (F2,91=0.05, p=1.000) hemisphere.
Hemispheric differences
The lesioned hemisphere of the AIS group showed lower FA (t21=3.68, p=0.001, d=-0.817) (Figure 4) and higher MD (t21=4.10, p<0.001, d=0.425) compared to non-lesioned hemisphere, whereas children with PVI showed higher MD (V28=330.00, p=0.02, d=0.558) in the lesioned hemisphere compared to non-lesioned, but no between hemisphere differences in FA (t28=0.22, p=0.829, d=-0.282). Interhemispheric differences for the control group were not significant for FA (t42=1.97, p=0.06, d=-0.302), but did show higher MD in the non-dominant (right) hemisphere (t42=4.95, p<0.001, d=-0.755).
Along the tract group differences
Consistent differences throughout the tracts were observed in the lesioned hemisphere (Figure 5B). FA was lower in the AIS group (p<0.001) compared to TDC along the entire tract (ROIs 2-10), whereas FA in the PVI group was lower in the anterior portion of the tract (p=0.03 to p<0.001) at ROIs 0-4 and again at ROI 7 when compared to TDC. Children with AIS had lower FA at ROIs 3 and 5-10 (p=0.008 to p<0.001) in the lesioned hemisphere and lower FA at ROIs 1, 7 and 8 (p=0.01 to p=0.03) in the non-lesioned hemisphere when compared to children with PVI. In the non-lesioned hemisphere (Figure 5C), differences in FA between stroke and TDC groups were observed at several locations. FA in both AIS and PVI was lower (F2,88=4.19, p<0.001) compared to TDC at the optic chiasm (ROI 0). PVI showed lower FA compared to TDC anterior to the LGN at ROI 2 (F2,88=5.48, p=0.004), whereas AIS showed lower FA at ROI 4 (F2,88=0.49, p=0.03), and ROI 8 (F2,88=1.45, p=0.005) when compared with TDCs.
Children with AIS showed higher MD throughout most of the posterior portion of the tracts at ROIs 4-10 (p=0.009 to p<0.001) in the lesioned hemisphere and the non-lesioned hemisphere (p=0.01 to p<0.001 at ROIs 1, 3 and ROIs 7-9) compared to TDCs. Children with PVI had higher MD (p=0.03 to p<0.001) at ROIs 0, 6 and 10 compared to TDCs. There were no significant differences in MD between participants with PVI and AIS in the non-lesioned hemisphere, AIS had higher MD (p=0.02 to p<0.001) than PVI at ROIs 4-10.
Between hemisphere differences of AIS and PVI groups along the tract
Participants in the AIS group had significantly lower FA and higher MD (between p<0.01 and p<0.001) in all sampled subsegments along the entirety of the tract in the lesioned hemisphere compared to the non-lesioned hemisphere. In the PVI group, between hemisphere differences of lower FA and higher MD (between p<0.02 and p<0.001) were found in the LGN and the sections just anterior and posterior to the LGN (ROIs 4 through 7), but no interhemispheric differences existed along other parts of the optic tract or optic radiations. TDCs showed higher FA (p<0.001) and lower MD (p=0.001) in the LGN of the left hemisphere (ROI 5,), and lower MD in the optic radiations of the right hemisphere (ROIs 6-9, p-values between p=0.007 and p<0.001).
Between hemisphere comparison within AIS group
There were no differences in FA in the lesioned (t18=2.00, p=0.061, d=0.892) or non-lesioned hemisphere (t18=1.34, p=0.198, d=0.597), nor MD in either hemisphere (lesioned t18=0.087, p=0.932, d=0.04, non-lesioned t18=1.250, p=0.430, d=0.506). In both AIS and PVI groups, children with left hemisphere injuries (mean 51.2cm3) had larger lesion volumes than right hemisphere lesions (mean 32.2cm3).
Discussion
Our results confirmed our hypothesis that the structural development of the visual white matter pathways is more likely to be altered in children with AIS, showing lower FA and higher MD, when compared to PVI and TDCs. Consistent differences in diffusion microstructure characteristics between the AIS, PVI and TDC groups were found in both the lesioned hemisphere and the seemingly intact non-lesioned hemisphere. Along the tract analyses suggested varying microstructural alterations along the visual tracts between the AIS and PVI groups.
Perhaps the most interesting findings are the microstructural alterations that occurred in the non-lesioned hemisphere, with the most significant differences observed in the AIS population. This is consistent with previously identified alterations in the non-lesioned hemisphere of children with perinatal stroke, including differences in myelination,10 structural changes in the thalamus,7 diffusion properties of the corticospinal tract,30 cerebellum,31 basal ganglia,32 cortex,9 and widespread alterations in the white matter connectome.8 These alterations have been associated with type and degree of disability, suggesting clinical relevance. While previous studies have focused on the development of the motor system and motor outcomes, our results demonstrate that perinatal stroke may also affect the development of the afferent visual pathway in non-lesioned hemisphere. This is suggestive of whole-brain plastic (re)organization that occurs following early-life injury, and that white matter structural differences are also occurring in the non-lesioned hemisphere. The addition of clinical visual outcomes such as perimetry and perceptual assessments would help determine the clinical implications of these developmental changes. Future comparisons of visual and motor pathways within subjects could also provide information on potential interactions between the two systems, and its effects on functional outcomes.
Our comparison of visual pathway white matter microstructure showed lower FA and higher MD in both PS groups compared with TDCs, with the most striking differences occurring in the AIS group. Our age-matched TDC sample is comparable with current literature of whole brain white matter development33 and provided a template with which to compare the results of the PS groups, with injuries likely to have damaged visual pathways and/or disrupted typical development of these pathways and related broader brain networks.4 In our study, both the AIS and PVI groups showed significant alterations in FA in both the lesioned and non-lesioned hemispheres compared to TDCs. As perinatal stroke can occur between 20 weeks gestation and 28 days of postnatal life,3 at least some of these observed microstructural differences in the visual tracts of PS participants likely reflect subsequent developmental alterations, rather than pathway destruction that would seem more likely in the static brains of adults with stroke. Developmental alterations could include cohesiveness and compactness of fibre tracts, membrane permeability or differences in axonal packing, although we are unable to specify which underlying mechanisms are occurring in this study.34,35 These findings add to the body of evidence that describes alterations from typical development after PS, such as white matter differences in the motor system and their association with motor disability.10,30 Future inclusion of visual and perceptual outcomes are needed to determine the association between these white matter differences and the level of functional visual performance.
The differences between AIS and PVI groups, with the AIS population showing the most significant alterations, could also be a result of lesion timing as has been previously identified.1 PVI is thought to typically occur mid-gestation, before myelination of the optic radiations has begun.36 In the sensorimotor system, white matter tracts may alter “around” such lesions in children with PVI.30,37 In contrast, AIS usually occurs near birth when damage to the visual pathways and surrounding areas may limit the ability of the brain to “re-build” or “re-route” damaged tracts.38 White matter development of the visual pathways typically increases sharply in infancy and slows around age 6 when it starts to resemble the adult system.39 Injuries occurring earlier in development might allow for a longer period of post-injury white matter development to occur in the visual system. As PVI occurs during mid-gestation when the visual system is less developed, this could create an additional window for developmental reorganisation after insult that is less available to children with AIS.40,41 Another consideration is the increasingly understood vulnerability of the white matter to “development arrest” during the window of extreme prematurity where alterations in the maturational sequence of oligodendrocytes are a primary contributor to premature white matter injury.42 Although all our PS participants were term birth, they still show evidence of this altered white matter microstructure. An interaction amongst these or other mechanisms may explain the differences we observed between AIS and PVI populations.
Lesion location may also play a role in the differences in the visual pathways we observed between stroke groups. Damage to post-geniculate pathways often seen in AIS has been linked to structural alterations and functional differences.6,43 Children with middle cerebral artery lesions may also see damage to subcortical structures, where afferent visual information is relayed through the optic radiations to the visual cortex. PVI injuries are typically limited to the periventricular white matter, often leaving the subcortical structures undamaged.44 In children with AIS the lesion often affects white matter and impacts the typical pathway of the optic radiations.3 All the AIS participants in our sample had sustained MCA strokes, resulting in lesions posterior to the LGN and often affecting the typical pathway of the optic radiations. Larger and more distal arterial occlusions were also seen to affect the optic tract between the optic chiasm and LGN. PVI lesions appeared to have less of an impact on the white matter microstructure posterior to the LGN and differences were mainly seen anterior to the LGN.
Along tract statistics were used to quantify the microstructure of these different stroke types. Specifically, for the AIS group, the entire visual tract from the optic chiasm to the primary visual cortex showed lower FA and higher MD than typically developing peers. Since AIS lesions were caused by MCA infarcts in our sample, the vascular territory of these arteries covered a wide area of brain and encompassed significant individual variability. This projection of degeneration is illustrated in Figure 5 where microstructural measurements are different and more variable for the AIS group in areas remote from the primary lesion. This damage distant to the original lesions, known as diaschisis, is present in some children and not in others. Along tract statistics also show alterations in microstructure in the optic tract between the chiasm and the LGN, which has not previously been identified in the AIS population and is suggestive of retrograde degeneration from the lesion site. Along tract statistics show additional evidence of diaschisis extending away from the primary lesion and affecting areas that are functionally and structurally connected to that lesion.
Multiple limitations of our study are considered. Head motion in children is typically higher than in adults, resulting in a greater number of artifacts and can contribute to image degradation. Head motion has also been shown to be higher in children with brain injury,45 although there are no studies on head motion specific to the PS population. Head motion between groups was not measured in this study but participants with significant head motion were excluded from our analysis. The acquired MRI data did not include any reverse phase encoding sequences and thus a synthetic b0 was generated. Placement of ROIs was completed manually. Automation of this process may be useful in larger sample sizes, but may also be challenging due to the structural variability of PS brains based on lesion location and size. The diffusion metrics used here such as FA and MD are voxel specific and thus susceptible to the issue of crossing-fibres, particularly in areas such as the lateral geniculate nucleus, through which our tracts pass. While conventional thinking may suggest that a higher FA reflects greater white matter density, interpretation should consider that factors such as fibre diameter, density, myelination, and membrane permeability may all affect the restriction of water diffusion.34,35,46 Although we may wish to make an inference regarding connectivity, we are only able to conclude that white matter microstructural alterations are present in children with PS compared to our control group. The addition of functional visual data would provide information about whether these microstructural differences are related to functional visual outcomes. Children with PS in our population all had motor impairment, specifically symptomatic hemiparetic CP, creating a selection bias for larger and centrally located lesions that may limit generalizability to the entire PS population.
Conclusion
This study contributes to existing evidence that whole brain re-organization occurs after PS, including the afferent visual pathways. Our analysis is the first to identify microstructural changes in the optic tracts and optic radiations exclusively within the PS population. Additional clinical and functional outcomes in this population is required to advance our understanding of these structural changes, and the effects of developmental plasticity on visual outcomes in children with early brain injuries. Understanding the impact of these microstructural changes on function could improve clinical practice and lead to development of novel assessment and therapeutic techniques to maximize the plasticity of the developing brain to optimize outcomes for children affected by PS.
Data availability statement
Data is available upon establishment of a formal data sharing agreement, approval from the authors’ local ethics committee, and approval from the requesting researcher’s local ethics committee.
Funding sources
This research was funded in part by a project grant through CHILD-BRIGHT, supported by the Canadian Institutes of Health Research. Meghan Maiani is supported through the Cumming School of Medicine “Vision and stroke recovery across the lifespan” grant.
Conflict of interest disclosures
The authors declare that they have no competing interests.
Acknowledgements
We thank the participants and their families for making this research possible.

_due_to_middle_cerebral_a.png)


_ten_sample_locations_along_the_tract_starting_from_the_optic_chiasm_(roi_0)__through_th.png)
_due_to_middle_cerebral_a.png)



_ten_sample_locations_along_the_tract_starting_from_the_optic_chiasm_(roi_0)__through_th.png)